

Levulinic acid
Levulinic acid, or 4-oxopentanoic acid, is an organic compound with the formula CH3C(O)CH2CH2CO2H. It is classified as a keto acid. This white crystalline solid is soluble in water and polar organic solvents. It is derived from degradation of cellulose and is a potential precursor to biofuels, such as ethyl levulinate.
Levulinic acid was first prepared in 1840 by Dutch chemist Gerardus Johannes Mulder by heating fructose with hydrochloric acid The first commercial production of levulinic acid began as a batchwise process in an autoclave by starch manufacturer A. E. Staley in the 1940s.
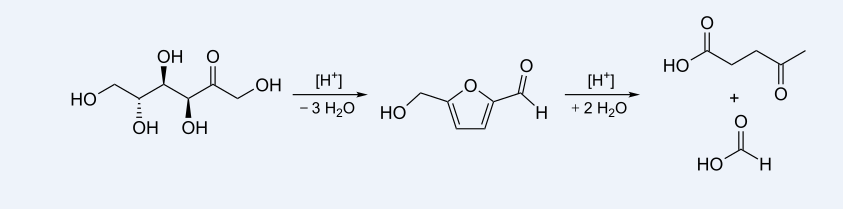
In 1953 Quaker Oats developed a continuous process for the production of levulinic acid. In 1956 it was identified as a platform chemical with high potential. and in 2004 the US Department of Energy (U.S. DoE) identified levulinic acid as one of the 12 potential platform chemicals in the biorefinery concept.
The synthesis of levulinic acid from hexoses (glucose, fructose) or starch in dilute hydrochloric acid or sulfuric acid. In addition to formic acid further, partly insoluble, by-products are produced. These are deeply colored and their complete removal is a challenge for most technologies.

Many concepts for the commercial production of levulinic acid are based on a strong acid technology. The processes are conducted in a continuous manner at high pressures and temperatures. Lignocellulose is an inexpensive starting material. Levulinic acid is separated from the mineral acid catalyst by extraction. Levulinic acid is purified by distillation.
